6.8 Microalgae for High-Value Bioproducts
By Joel L. Cuello, Professor, Department of Biosystems Engineering, University of Arizona
Current research and engineering innovations for a potential microalgal high-value products industry in southern Arizona to be linked with external bioeconomy nodes to achieve circularity.
Commercial large-scale production of microalgae began in the late 1960s in Japan, then spread throughout the globe in the 1970s and 1980s. In recent years the number of commercial large-scale facilities around the world has increased at a nearly exponential rate as demand for animal feed, nutraceuticals, vitamins and lipids, biofuels and bioplastics has increased.
The global production of microalgal biomass was estimated to be more than 5,000 dry tonnes in 2005 with a value of more than U.S. $1.25 billion, which excluded the value of processed products (Spolaore et al., 2006). Annually, about 3,000 dry tonnes of Spirulina are produced in China, India, Myanmar, the United States, and Japan; 2,000 dry tonnes of Chlorella in Taiwan, Germany, and Japan; and 1,200 dry tonnes of Dunaliella salina in Australia, Israel, the United States, and China (Spolaore et al., 2006). In 2008, the global production of microalgal biomass was estimated to have reached 9,000 dry tonnes per year (Benemann, 2008).
There are two general approaches in the mass cultivation of microalgae. Open systems (such as open ponds or raceways) are economical to build and operate; however, they have major disadvantages, including significantly high contamination risks, fluctuations in environmental conditions, and significantly lower and less reliable productivity. Closed systems (such as photobioreactors), though relatively costlier, have the advantages of control of environmental conditions, significantly lower risk of contamination, and higher productivity and reliability than open systems.
The photoautotrophic growth of microorganisms or cells is enabled by the photosynthetic capacity of the chlorophyll-containing microorganisms or cells, whereby carbon dioxide (CO2), through photosynthetic carbon fixation, serves as the carbon (or food) source. Photoautotrophic growth requires the presence of light for photosynthesis to occur. A steady supply of CO2 when light is available also promotes culture growth.
Heterotrophic growth, by contrast, takes place when the microorganisms or cells, in the absence of photosynthetic CO2 fixation, rely on exogenous carbon-based molecules, typically sugars such as glucose or sucrose, present in the liquid culture medium as their carbon (or food) source. This mode of growth also requires a steady supply of oxygen (O2) which the microorganisms or cells need as they breakdown the carbon-based molecules through the process of respiration. Since light is not essential, heterotrophic production is generally carried out in darkness. Mixotrophic growth takes place when the microorganisms or cells grow both photoautotrophically and heterotrophically.
The University of Arizona Biosystems Engineering Laboratory in the Department of Biosystems Engineering has been building a growing portfolio of patented/patent-pending originally designed scalable bioreactors with superior mixing or hydrodynamic characteristics, if not also with modular design and lower-cost, for the scaled-up cultivation of microalgae cultures as well as other microbial cells (Figure 1). The high-value products generated include proteins for human consumption, animal feed, nutraceuticals (e.g., omega-3 fatty acids), lipids for biofuels, cosmetic ingredients and vitamins, among others.
Southern Arizona represents a competitive location for the establishment of a microalgal high-value-products industry owing to the availability and relatively low cost of land as well as the year-round abundance of solar radiation, among others. Table 1 shows how a microalgal high-value-products industry in southern Arizona could be linked with external bioeconomy nodes to achieve circularity. Potential input feed could come from external industry nodes, including electric power plants, municipal wastewater facilities, greenhouses, open-field agriculture, aquaculture operations, and food and beverage manufacturing facilities, among others.
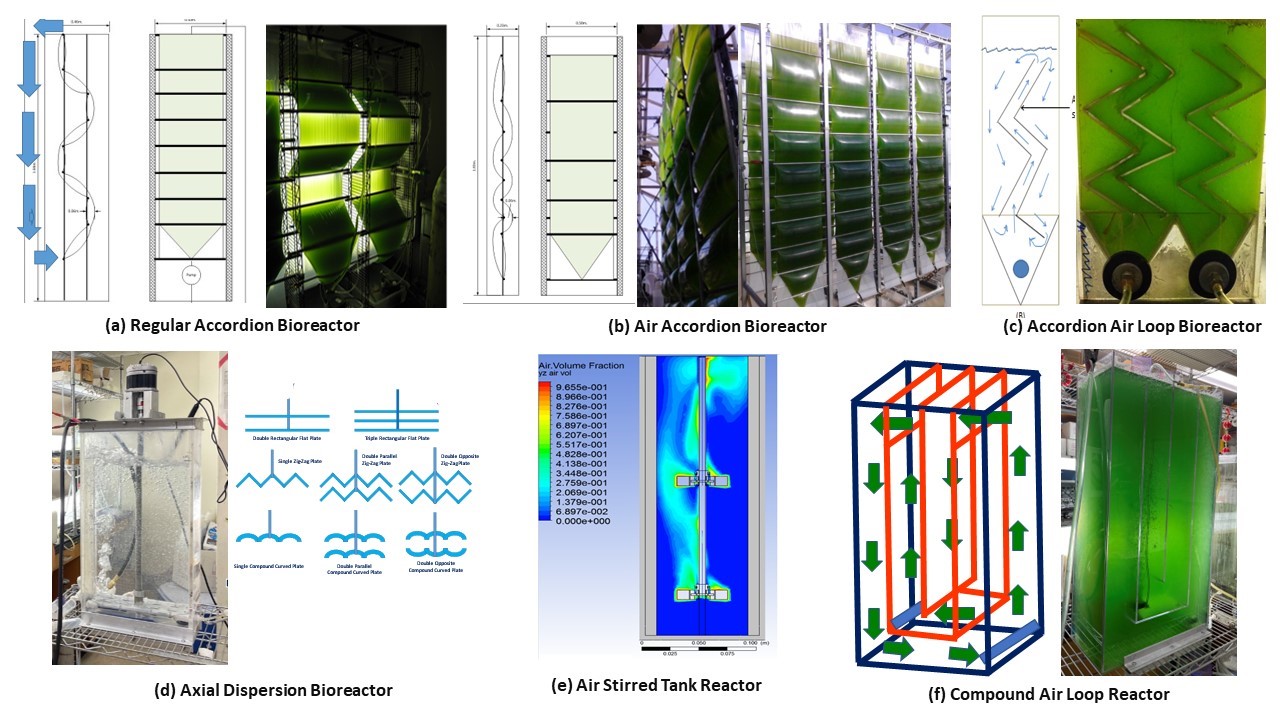
Figure 1. The University of Arizona Biosystems Engineering Laboratory portfolio of patented/patent-pending originally designed scalable bioreactors with superior mixing or hydrodynamic characteristics, if not also with modular design and lower cost.
Table 1. Scaled-up cultivation in bioreactors of microalgae cultures for high-value products.












